The Changing Schedule
A visual comparison of vaccine schedules: 1982 vs. 2016.
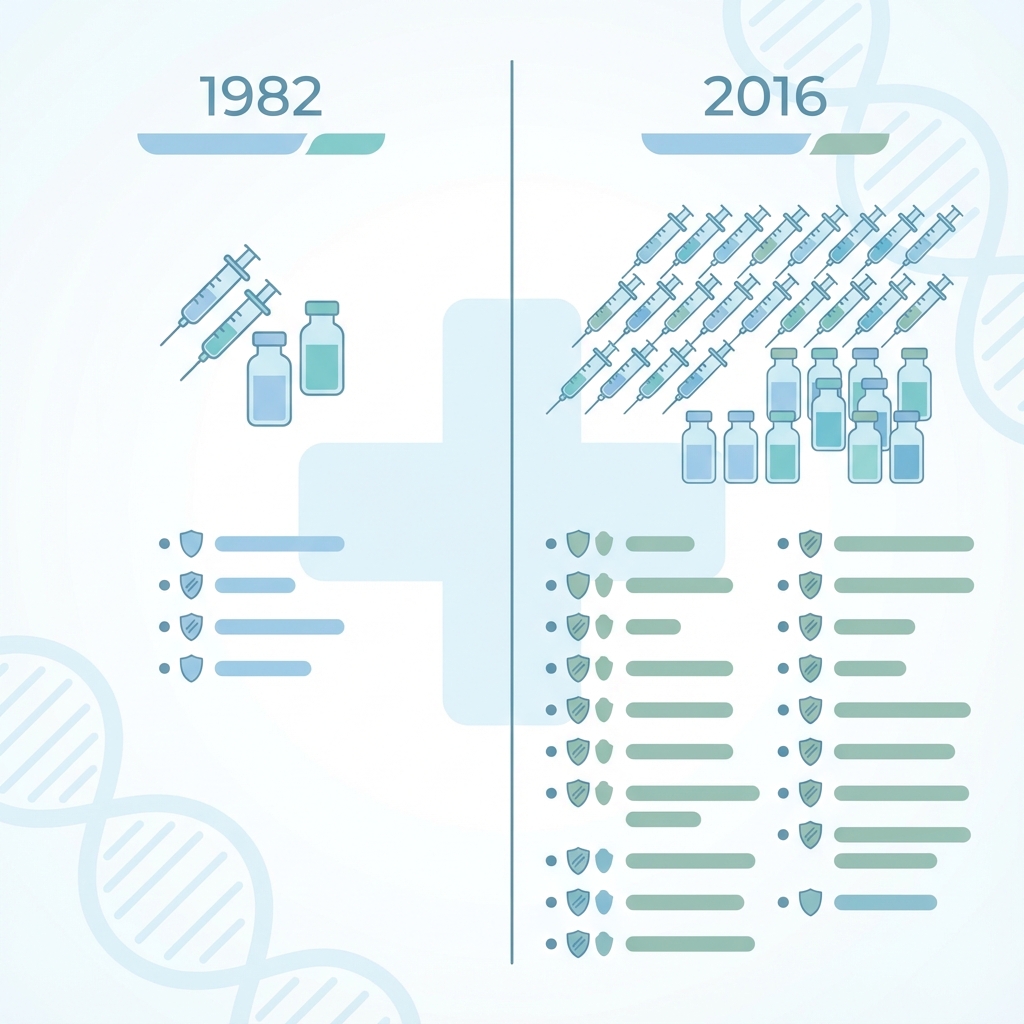
The dramatic increase in the number of doses children receive today compared to 1982.
Click images to enlarge.
Empowering families with information, studies, and direct links to key organizations.
On June 24, 2020, we provided testimony to the Advisory Committee on Immunization Practices (ACIP). This touches on the critical safety concerns observed in cases like Norah's.
The Vaccine Adverse Event Reporting System is co-managed by the CDC and FDA. It is the primary mechanism for reporting vaccine side effects.
Research into Sudden Unexpected Death in Childhood (SUDC) explores potential links between febrile seizures, hippocampal changes, and sudden death.
Information on the National Vaccine Injury Compensation Program, including how to file a claim and find specialized legal help.
The National Childhood Vaccine Injury Act of 1986 established the current "no-fault" system, shielding manufacturers from liability.
A visual comparison of vaccine schedules: 1982 vs. 2016.

The dramatic increase in the number of doses children receive today compared to 1982.
Click images to enlarge.